Site news will appear here.
2025-09-24:
My web host will be performing necessary server maintenance October 1st from midnight to 4:00 a.m. PDT.
During this time, the atomic-spectra site may be temporarily unavailable.
I am still looking for a full time role if anyone reading this knows of an opening in their organization,
either remote or located in the Phoenix, AZ area.
It doesn't have to be computer related.
Résumé available on request.
2025-08-26:
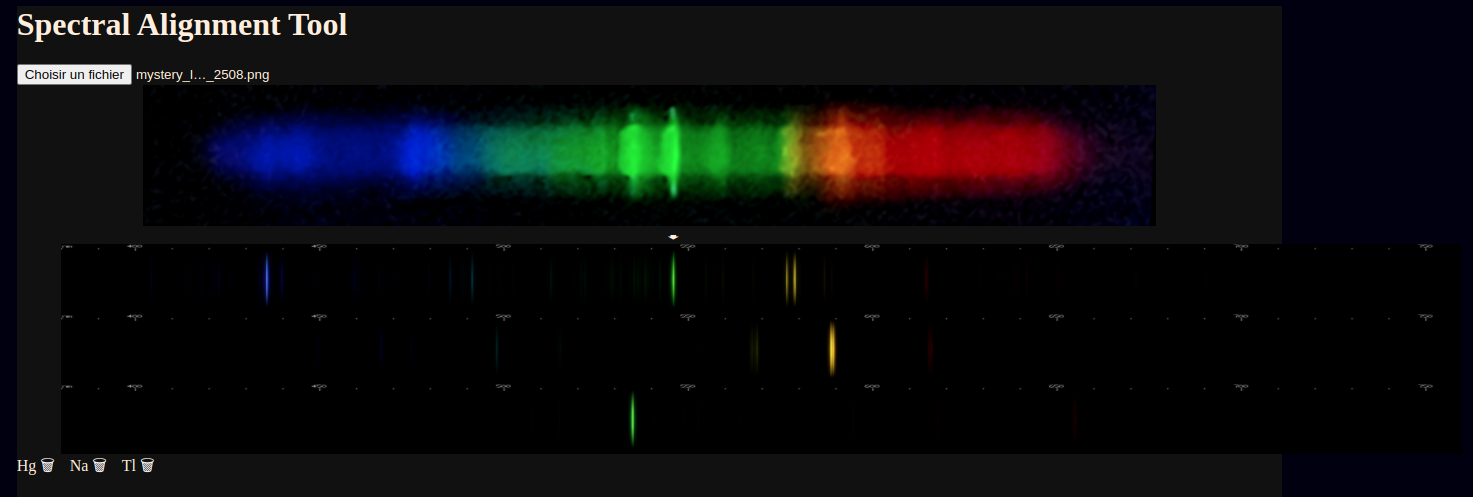
New website feature! One that can be left enabled indefinitely since it runs entirely client side.
This page lets you select an image file (no upload - preview only)
and then line it up with known spectra of elements.
There's a description of how it works here
along with a whole new spectrum I've yet to be able to fully identify... perhaps you can solve the mystery!
I am still out of work but staying optimistic and submitting applications.
Nothing is definite yet.
Once I get working again then I can hopefully return to dedicated hosting and reenable site features
like wavelength tables and Grotrian diagrams, besides overall better website performance.
Until then, if you are looking for someone proficient in C++/PHP/JS/SQL/HTML/CSS
for a full-time or contract role,
please don't hesitate to reach out -
résumé available on request.
2025-08-01:
Just a small update. First a little good news,
it appears the code change made back in March has improved this website's performance,
as I've not had to reboot the virtual server very often (it used to be an almost daily thing).
Site responsiveness still isn't quite as fast as I would prefer,
especially knowing a lot of people rely on being able to access these spectrum images.
Also some features I really wanted to keep, such as the wavelength tables and Grotrian diagrams,
are unfortunately too resource intensive to enable right now.
Up until May of 2024 this site was on a dedicated hosting plan that facilitated a much faster website response
and allowed all the fancy pretty features to be active,
but at about 20 times the price.
Since then I have been out of work without the income that used to make it possible to afford the upgraded hosting,
and am now driving for rideshare which isn't enough for even basic living expenses.
I owe 9.5 months of back rent and the electric bill is also overdue.
This isn't a fundraiser message (give someone a fish etc),
however if anyone reading this is looking for a software/web developer proficient in C++/PHP/JS/SQL/HTML/CSS
for a full-time or contract role,
please let me know.
Résumé available on request via julie [at] umop *dot* net.
2025-03-14:
Happy Pi Day!
Last year I had to downgrade my hosting to a shared virtual server due to a reduced budget,
and the shared server has been struggling to serve requests during peak usage times.
It has been difficult trying to find the exact cause of the poor performance.
Today I have implemented a change that will hopefully improve website functionality,
and I will be monitoring the site, when possible, over the coming days to see if everything stays up.
Please feel free to keep using it as you normally would.
2024-09-29:
Check out this video by Project 326 featuring atomic-spectra.net.
In fact, do yourself a favor and subscribe to the channel, there are a lot of really interesting videos on there!
2024-09-27:
Thank you!!!!
I want to thank everyone for all the messages of support and the generous donations. I am touched that my website is important to many many people and delighted that my scientific efforts are beneficial to the world. My personal financial situation is still dire, but your donations are keeping the website running. Thank you!!!
2024-08-10:
We have identified and fixed a performance issue in the Interactive Viewer.
It should now behave much more smoothly without so many excessive page load times and server overload errors.
2024-01-18:
The site admin's financial situation has recently taken a turn for the worse, and it may be a while before things are back to normal again.
I have added a donation button through buymeacoffee.com.
I'm also #opentowork.
Any donations are completely optional and graciously appreciated.
All content on this website is freely available, i.e. no paywalls of any kind, and all spectrum images are in the
Public Domain/CC0 unless otherwise noted.
And it's going to stay that way.
This website is a source of scientific information, and scientific information should always be free.
2023-07-04:
Professor Davis a.k.a. ChemSurvival has made a great video about the colors of fireworks,
featuring some spectrum images from here on Atomic-Spectra.net.
Make sure to check out his YouTube channel
and subscribe for lots more educational content about the fascinating field of chemistry!
You may also have noticed our flag counter at the bottom of the page.
We are proud to offer free information to the scientific community worldwide!
However, the data for the flag counter are skewed,
as we get a large number of hacking attempts from malicious bots located in specific regions.
These bots are relentless but their attempts are in vain, since we have nothing their owners want.
Nonetheless, it is always good to be able to waste the time and resources of cybercriminals.
2023-06-23:
We have a brand new shiny domain name: Atomic-Spectra.net!
The old domain will still work and will display the same information.
2022-09-03:
We've been published! Be sure to check out these element spectra in
Algebra the Beautiful: An Ode to Math's Least-Loved Subject
by G. Arnell Williams.
Coders Wanted! Our sister project, PrimaryOdors, is volunteer collaborators in developing and maintaining
a more advanced molecular docker. Find it on GitHub.
2022-01-09:
Check out our sister project, PrimaryOdors.
Its purpose is to study human olfaction and the encoding of odor perception from the patterns in which aroma molecules excite the receptors in the nose.
2021-07-01:
The Interactive Viewer now has a new mode: if the wavelength range is set to 400-1050nm, it simulates the spectrum as a
Raspberry Pi NoIR camera might register it.
2021-06-18:
Each element's spectrum is unique, like a fingerprint.
But some element spectra are near doppelgangers when observed visually.
So they're different. But they're similar. But they're different. But they're similar.
Nitrogen and lead, for example, have an uncanny visual resemblance, as do copper and chromium.
About a dozen elements' spectra can be described as a dense swath of green lines and a dense swath of blue/violet lines.
Each of these elements have their lines at slightly different wavelengths, so can be readily distinguished in
an expensive spectroscope, but the eye does not measure wavelengths so precisely.
Most of the elements in the Interactive Viewer now include links to their closest lookalikes,
making it easy to compare and contrast spectra that are similar in appearance.
2021-06-18: For some reason, hackers think it's worth their while to try and break this website.
It's your standard issue SQL injection stuff, basically the laziest kind of hacking that every website should protect itself from at bare minimum -
and a great many sites don't!!!
Peeking at the logs a few days ago I found this gem:
95.217.250.107
2021-06-13 12:27:26
spectrum.php?elem=N Ior 0=0``/">'/*!13337or*/'256'='4'
Yeah n00b, you think you're leet. But you're not. 😂
2021-05-23: In the Interactive Viewer, most spectra that have a photo now have a link underneath that reads "expand".
If you click on it, the photo will automagically resize and reposition itself to line up with the generated lines image.
2021-05-20: Over the past few days, I've significantly upgraded my camera setup and taken much more detailed photos of many of the element spectra.
A few of them (notably Mn, Pr, and Nd) show a definite variance from the old photo.
Not sure why this is, but there's every reason to trust the new detailed photos vs. the old blurry ones.
Due to work obligations, social plans, and health conditions, it may take me a while to get everything in sync to the better data:
the photographic periodic table for instance, and the guide for learning to identify spectra.
The periodic table is also on Wikimedia Commons,
along with my photos of individual element spectra, most of which have to be updated, so that is also part of the same workload.
2021-05-15: The new flash cards are now provided on their own for memorization;
no pressure to answer any cryptic "what element is this" screens, and you can exit at any time.
2021-05-10: Here's a new guide for recognizing spectra visually.
Sure, there are almost 100 spectra to become familiar with, but this will greatly simplify the ones you're likely to ever encounter.
It starts off simple and makes clarifying comparisons and descriptions all the way through.
2021-05-08: Someone recently contacted me and said that people's lives were at risk because this site didn't have a privacy policy.
While I guarantee you that that claim is ridiculous hyperbole, I am sensitive to everyone's concerns about how user information may be stored or used.
Rest assured, this website does not collect personal information, or any information we could use to individually identify you.
Please view our Privacy Policy for more details.
2021-05-02: Great news! This site now offers line tables for all the spectra featured in the various pages and web apps.
It uses wavelength data from NIST's Atomic Spectra Database, combined with intensity adjustments to more accurately reflect the perceptual
brightnesses of visible lines. It also includes upper and lower energy level information for a great many lines.
2021-04-26: I have arranged my spectrum photos onto the periodic table. You can see the result
here. The photos have been aligned so that they're all on the same wavelength scale, and will match the lines in the normal periodic
table image if overlayed with it. Elements lacking a photo retain their auto generated spectra, with a small graphic to explain why the photo is missing, e.g.
fluorine and phosphorus are too reactive to handle safely, although there may be workarounds for one or both.
Regarding the elements I still have not photographed:
(click to see the list.)
- I have a sample of 33.33% fluorine/66.66% helium in a sealed glass ampoule. It appears to be at close to atmospheric pressure, therefore unsuitable for a gas discharge arc.
- No allotrope of phosphorus exists that I can simply generate a spectrum of, however there should be a way to devise an apparatus to run an arc through a very small sample of vapor at low pressure.
- Metallic rubidium and cesium are more dangerous than my setup allows, but I might at some point do a flame test of their chlorides.
- Thallium is very very toxic, and both my sources are fully in agreement on the intensities of its lines. Its Grotrian diagram also confirms what my sources say. There is no reason to photograph thallium.
- There is a supplier that offers technetium, but at a high price and probably not enough quantity to get a good spectrum photo.
- It is also possible to get americium, but again in very small quantities, also it is in oxide form which is not suitable for my purposes.
- Radium is available but only as a sulfate and therefore also unsuitable for spectrum photography.
Unfortunately, I do not have a setup for reducing tiny amounts of an insoluble salt of a reactive cation to pure metal.
Further, the calcium, strontium, and barium all gave me miniature fireworks shows when I took their spectra, and it would be dangerous for a radioactive metal to do
the same thing, even though all my experiments with radioactive substances are done in a safe enclosure.
- I have a sample of thorium, however it is very small, very brittle, very expensive, and covered in an oxide coating that precludes spectrum photography.
Hopefully I will dream up some kind of workaround. If you can think of a solution, please send me an email.
- Promethium, and the elements from polonium onward, are too unstable and not available for purchase, with a few exceptions, most notably depleted uranium which does have a photo on here.
2021-04-11: The Identification Game has been revamped with easier hints and a range of difficulty levels. It's a great way to learn to recognize the spectra, and the hints are always
available so you're never stranded on an unfamiliar spectrum. And if you win, there's a cool surprise at the end.
2021-03-31: I have procured samples of most of the elements and added photos of their spectra, in order to verify the relative intensities of the brightest lines.
The line intensities from my source data have been adjusted accordingly. I still have a few gas tubes to obtain, which should not differ much from the current data.
I am also looking into rare and hard to find metals as my budget allows. The samples have to be held by two ordinary alligator clips so I can spark them and take
the photos. As my remaining not-yet-ordered metals are all very expensive, they cost a lot for even the small quantities necessary for my experiment, so this part is
taking me longer than I would have liked.
2021-03-23: I have noticed a lot of people are still using the old legacy image on their sites. (Understandable, since it existed unchanged for several years.) There's
no reason you can't, just be aware it has some huge inaccuracies in it. If you have a copy of the old image, please consider replacing it with a current one. You can still
find the old image here if you so desire. Meanwhile, the final image will be ready before too long,
and it will differ very little from the current image.